Table of Contents
- What is Document Control?
- What Types of Documents in Quality Processes Need to Be Controlled?
- Navigating the Challenges of Document Control
- Key Components of the Document Control Process in a QMS?
- How the Document Control Process Works: A Real-Life Use Case in Action
- A Digital QMS: Ensuring Accessible, Secure, and Up-to-Date Quality Documents
Step into a smarter digital workplace
Get a Free Product TourWhat is the cost of an inefficient document control system in terms of time, money, and errors?
Imagine if the wrong version of a quality document is referred to during production or testing; what happens to your quality assurance efforts?
While maintaining quality allows businesses to establish trust in their brands, adhering to regulatory compliance reinforces that trust with formal assurance. Yet, the critical process of document control—managing records and documentation to confirm compliance with regulatory requirements—often remains underappreciated.
For sure, documents make up the core of your daily business transactions. From incident reporting and audit to employee training and vendor selection, every detail is recorded in the form of documents. Certainly, it eases communication, contributes to organizational knowledge, and records the decisions taken during the quality control and management workflow.
What is document control, and what are its core components? Is it different from document management, and how does it contribute to your quality management goals? Let’s find out!
What is Document Control?
Document control is a systematic approach that ensures accuracy, consistency, security, accessibility, and disposal of the documents created within the organization.
Now, this is not the same as managing documents.
Indeed, the concept of document control is often confused with document management. However, there are significant differences. While document management inclines more toward organizing and managing documents, document control is more concerned with ensuring the accuracy, security, and traceability of the documents. While the first includes creating, storing, classifying, and archiving documents, the latter keeps track of document access, distribution, versioning, controls, and approvals.
Although document control practices remain the subset of the entire document management concept, both are necessary when it comes to setting a robust stage for quality management.
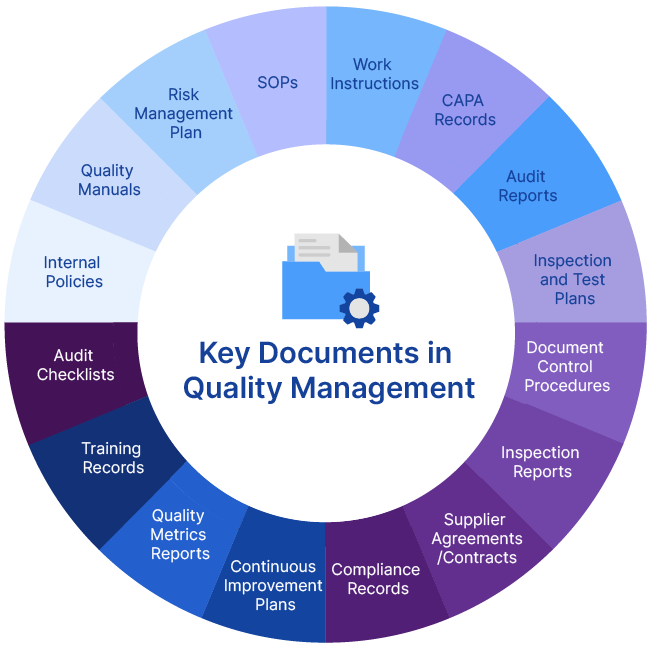
What Types of Documents in Quality Processes Need to Be Controlled?
Certainly, organizations deal with tons of documents daily. A document is created every time a workflow is planned or executed. It can be a form that identifies a quality issue or a manual or SOPs instructing the right approach to the employees. It can be an agreement created at the time of vendor onboarding, or a report that presents the effectiveness of the CAPA process.
These documents play a fundamental role in planning, implementing, and auditing quality management operations and allow cross-functional teams to exchange critical information and collaborate easily. However, these documents are vulnerable to mistakes, delays in manual routing, security breaches, misplacement, and the risk of being outdated. Here emerges the need for a robust document control process.
Navigating the Challenges of Document Control
1. Document overload
Modern businesses are facing a significant information overload. A recent survey reveals that 83% of respondents feel the rapid pace and increased connectivity in business is requiring them to produce, share, manage, and distribute more documents than ever. Such a number of documents created daily within the organizations leads to mismanagement of files and information silos, slowing down employee productivity and reducing operational efficiency.
2. Document accessibility in remote workplaces
According to the same survey, employees spend 59% of their productive time looking for misplaced documents. Document accessibility is another major concern that needs a digital document control strategy, especially for organizations working with globally distributed teams. Also, without a centralized document control system, organizations end up with either siloed file cabinets or cluttered network drives, resulting in miscommunication, errors, and delayed decision-making.
3. Document integrity
What if key documents are accessible to unauthorized people within the organization? Certainly, this can disrupt the document’s integrity. Along with accessibility, there comes a challenge of protecting document integrity. However, limiting document access manually can be challenging while working with cross-functional teams, specifically in a remote environment. When different members collaborate on the same file, they edit, add, and modify the document multiple times, raising the question of transparency and accountability.
4. Regular updating of documents
The quality management workflow involves several approvals and reapprovals of a single document, ultimately creating multiple versions of the same document. Furthermore, documents like policies, compliance documents, and training manuals are subject to updating with changing laws and regulations. Manually updating documents and managing their older versions can be time-consuming, cause human errors, and increase the risks of non-compliance.
Key Components of the Document Control Process in a QMS?
1. Document creation and development
The document control process starts as soon as a document is created anywhere in the quality management ecosystem. So, having a digital document control system that can offer centralized and accessible document storage remains a priority.
However, certain crucial aspects, such as leveraging auto naming functionality, having a clear document identification number, and adding meta tags to the documents, help quality teams to systematically organize and access documents effortlessly at the time of need.
2. Document review and approval
It’s important that certain documents like policies, manuals, technical reports, and contracts are checked for accuracy before they are approved and distributed within the organization.
The documents in quality processes are subjected to various modifications, reviews, approvals, and reapprovals. And planning an optimized document routing workflow can smoothen the review and approval process, saving time and enhancing efficiency. With a QMS, you can automate this stage of document control. Furthermore, automated workflows empower organizations to record each stage systematically, which further reduces manual errors, promotes transparency, and fastens decision-making.
3. Document version control
It’s crucial to have the most updated version of documents to uphold quality standards. After so many reviews, modifications, and alterations, teams end up having multiple versions of the same document.
Maintaining version history empowers teams to systematically store previous versions and record changes like who made what changes and when. Certainly, document versioning not only improves traceability but plays a vital role in making teams audit ready.
4. Secure document distribution
Quality management processes often involve legal, technical, and financial documents that must be shared securely, without any risk of alteration or corruption. Ensuring secure document distribution preserves the integrity and confidentiality of sensitive information shared across organizations.
To mitigate the risks of unauthorized access, it is essential to manage platform access by assigning roles to users or defining controlled permissions for individuals or groups. This approach allows organizations to regulate who can edit, review, or delete documents effectively.
5. Document archive and retention
Quality documents, once reviewed and approved by the stakeholders, continue to be in use until they are amended or modified according to the new guidelines or regulations. However, as soon as a new version comes out, the older version must still be kept for compliance, reference, and audit purposes. This is known as archiving.
Secure, digital, and accessible repositories and relevant metadata columns when, coupled with a clearly defined document retention and disposal policy, can help you complete the document lifecycle that resonates with your compliance and regulatory requirements.

With the key components of document control in mind, it’s time to select the ideal QMS for your needs. Download our QMS Buyer’s Guide to discover the perfect solution.
How the Document Control Process Works: A Real-Life Use Case in Action
To understand the document control process flow, it is important to integrate it with a real-life quality management ecosystem. Consider a pharmaceutical manufacturing unit that recently identified quality incidents that demand a change in the existing production process. The highlighted change includes implementing a new automated mixing machine that automatically assesses new formulations and combinations for their tablet. Let us see what role a robust document control plays here.
- The quality team initiates a detailed change request and submits it to the Change Control Board (CCB), which is an internal committee with representatives from different departments, like quality assurance, production, engineering, etc.
- The change request form and the detailed report that suggests the reason for the change are logged into a centralized library, making it easily accessible to all the relevant stakeholders.
- The CCB is authorized to approve, modify, or even reject the suggestions of the quality team. However, after conducting a thorough impact and risk assessment, it was decided to install the new machinery.
- The existing manufacturing process and documentation must be updated according to the new instructions. All the relevant documents, such as Standard Operating Procedures (SOPs), work instructions, specifications, operator safety manuals, and quality protocols, were updated and reviewed for compliance.
- The document control system enabled teams to automate the routing workflow, trigger email notifications, and integrate e-signature for even faster decision-making.
- Once approved, the revised procedures and operational workflows were distributed to the manufacturing teams, including technicians, operators, and supervisors. Their training records were updated for future audits.
- The new automated mixture was installed. The updated SOPs, manuals, and instructions were followed during the process, and performance was tracked.
- The older versions of all the documentation were securely archived in a dedicated library. Relevant metatags were used adequately to make them accessible for future references and audits.
A Digital QMS: Ensuring Accessible, Secure, and Up-to-Date Quality Documents
In today’s landscape, implementing a digital document control system to centralize your quality documentation is no longer optional—it’s essential. Even a single outdated instruction can result in substantial losses of time, money, and hard-earned brand value.
BizPortals QMS, a SharePoint-based quality management system, brings together all the necessary features to implement a flawless document control strategy. From document creation to disposal, it offers a robust platform for your cross-functional teams to efficiently collaborate and optimize the document control workflow.
Effortlessly BizPortals QMS leverage core document control features such as document versioning, automated approval, dedicated repositories, activity logs, metadata integration, e-signatures, multi-level permissions, and more for enhanced efficiency and audit preparedness. Furthermore, it empowers users to create customizable templates for standard forms and reports to bring consistency across different modules and departments.
There are even more features waiting for you.
Explore the full potential of BizPortals QMS. Schedule a live demo, connect with our experts, and discover how BizPortals can contribute endlessly to your quality management goals.
Table of Contents
- What is Document Control?
- What Types of Documents in Quality Processes Need to Be Controlled?
- Navigating the Challenges of Document Control
- Key Components of the Document Control Process in a QMS?
- How the Document Control Process Works: A Real-Life Use Case in Action
- A Digital QMS: Ensuring Accessible, Secure, and Up-to-Date Quality Documents
Step into a smarter digital workplace
Get a Free Product Tour
