When it comes to compliance audits or quality investigations, how confident do you feel about your organization’s response? Does your CAPA process offer clear, step-by-step guidelines to empower your team during these critical moments?
Behind every quality product or service lies a series of relentless efforts to ensure compliance and elevate product excellence. Indeed, businesses find themselves under intense pressure to navigate audits and investigations, where even a minor miscalculation can lead to costly repercussions.
For sure, implementing an effective CAPA process is the core of the quality management strategy, which is subjected to regulatory requirements. An effective CAPA procedure empowers organizations to plan and execute appropriate actions to not only eliminate the issue but also prevent its recurrence.
Simply put, CAPA is like a health checkup for your organization’s quality process. While a health checkup identifies potential risks like high cholesterol, it also addresses existing issues like poor lifestyle before they become serious. Furthermore, it considers continuous monitoring to spot trends, diagnose the root cause, and foster a long-term health plan to minimize future risks.
Similarly, the CAPA process begins with identifying issues, enabling quality teams to evaluate risks and find their root cause, preventing recurrence. Furthermore, it provides for creating and implementing a thorough action plan while keeping a complete record of each stage for audits and future reference.
However, in this blog, you will get a step-by-step explanation of a CAPA process with the help of a real-world use case, along with some expert tips to help you navigate the CAPA process successfully.
What is a CAPA Process?
A CAPA (Corrective and Preventive Action) process refers to systematically planning, implementing, and documenting all the corrective and preventive steps taken to neutralize existing quality issues and prevent their recurrence. From identifying and investigating quality issues to outlining and implementing a robust CAPA, this comprehensive process is divided into several key stages and workflows.
To help illustrate this concept, let us review a use case where a medical device manufacturing company implements a structured CAPA process to ensure the safety and compliance of its blood glucose meter (a medical instrument used to measure blood glucose levels).
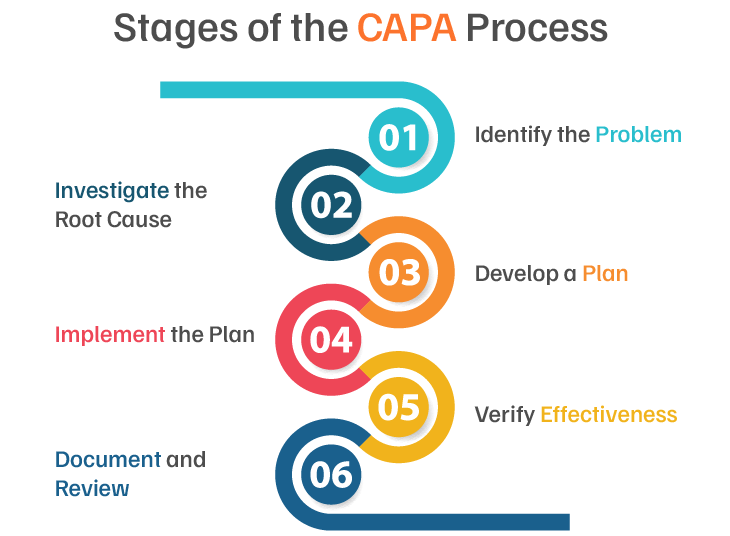
Navigating the Key Stages of a CAPA Process
1. Initial problem identification and logging
The CAPA process initiates when a significant quality issue is raised over the quality process. These quality issues can arise from various sources, including customer complaints, audits, internal monitoring, and quality reviews.
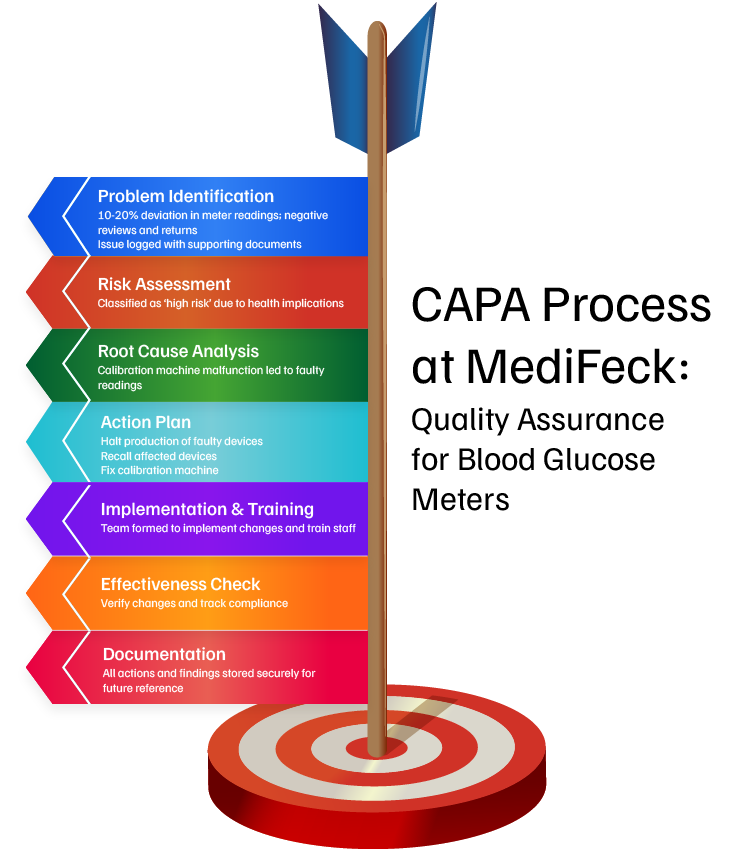
Such an issue occurred when the quality team at MediFeck discovered an exponential rise in negative customer reviews and product returns citing 10-20% deviation in the readings on blood glucose meter devices. After confirming the reviews, the team logged the issue with a detailed description and supportive documents, initiating a formal CAPA process.
Timely identifying and documenting issues can set the stage for a systematic risk assessment and enable teams to create appropriate action plans. Certainly, ready-to-use form templates with important fields like CAPA description, department, CAPA source, and more can aid quality teams in quickly reporting, logging, and tracking issues.
2. Evaluating risk and impact
Assessing the risk imposed by the specific incident is the next crucial step in the CAPA process. Indeed, this involves an in-depth analysis of the issue after carefully studying supportive documents and data collected from various sources.
This is demonstrated by the situation at MediFeck, where stakeholders formed a dedicated team to assess the severity associated with the faulty readings. As a blood glucose meter plays a vital role in managing blood sugar levels in patients, it can cause potential health risks. Based on this assessment, the incident was reported as ‘high risk’ and needed immediate attention to accelerate the CAPA implementation.
It helps organizations prioritize the issues based on their potential impact and aids in making informed decisions. However, in digital CAPA management software, a dedicated risk evaluation form allows teams to upload qualitative and quantitative findings, ensuring stakeholders are informed about the nature and potential impact of the issue.
3. Analyzing the problem to determine the root cause
Root cause analysis is a systematic process that identifies the fundamental reasons for the incident. Being an essential component of the CAPA process, it helps quality teams outline robust corrective actions and take appropriate preventive measures, ultimately leading to enhanced product quality and continuous improvement.
This was evident when quality assurance specialists at MediFeck analyzed the gathered data and detected a fault in the calibration machine as the root cause. A specific batch of sensors had been wrongly calibrated due to a malfunction in the calibration machine, resulting in faulty readings by the blood glucose meter.
Conducting a thorough root cause analysis is vital for identifying the fundamental reason behind the incident. However, unlike the traditional CAPA process, digital CAPA management allows users to study incident trends and data, track batches, identify the root cause, record findings quickly, and suggest measures from one place.
4. Creating a comprehensive action plan
Evidently, after finding the root cause of the incident, defining the objectives of the action plan will be the first crucial step. For example, at MediFeck, once the root cause was detected, production was stopped immediately. A thorough action plan, along with the supportive records, were documented and routed to the designated authorities for approval.
The corrective and preventive action plan made by the MediFeck CAPA team includes the following:
- Collaborate with the production team to stop the production of devices and revise the operating process.
- Affected devices must be recalled to prevent further harm.
- Faulty devices in the warehouse must be isolated.
- Fix calibration machines, update the maintenance schedule, and set deadlines to start production of error-free devices.
- Installation of a real-time calibration monitoring system on the production line.
- Enhancing the supplier quality control program with stricter calibration standards.
Assigning key responsibilities for execution, reviews, and approvals, deciding the timelines, and documenting the action plan can contribute to enhanced accountability and effectiveness of the CAPA process. Furthermore, managing the CAPA procedure digitally allows quality assurance teams to use centralized repositories to organize different components of their action plan, easily assign and track tasks, and leverage pre-built action plan templates to infuse consistency within the organization.
5. Implementing corrective/ preventive action
Effectively implementing a corrective and preventive action plan is a collaborative effort. From clearly defined, documented, and accessible protocols to proper training and regular follow-ups, it should be a streamlined process. As soon as the CAPA action plan is approved by the designated authorities, teams should be ready to play their role within their respective modules.
For example, at MediFeck, once the action plan was reviewed and approved, a team was formed to implement the suggested changes. The changes were integrated into the quality process, employees were trained according to the new standards, and each step was documented to ensure transparency and audit readiness.
Indeed, automating document routing, developing employee training modules (if necessary), tracking key metrics, and regularly monitoring progress are essential. However, features like built-in reporting and analytics, automated document approval workflows, electronic signatures, and automated email notifications can play a vital role in defining the success of your CAPA process.
6. Assessing the effectiveness of actions
As a crucial stage of the CAPA process, a timely audit ensures strict adherence to regulatory compliance and mitigates associated risks. Certainly, the audit procedure keeps a check on the results and the effectiveness of the actions executed to eliminate the root cause of the problem.
As in the case of MediFeck, the CAPA team conducted an audit to verify the implementation and effectiveness of the changes made to the process for new batches of their blood glucose meters. The new batches were tested thoroughly to cross-check them for the readings, and the data was recorded on the portal. The audit procedure checks the metrics opted to track the progress, verifies records and reports, and reviews the quality control logs to verify the steps taken. If all the readings are up to the mark, the issue can be marked as resolved and the CAPA process can be closed.
Documenting every step of the CAPA procedure, maintaining a detailed and accessible CAPA log, enabling version control and audit trails, and leveraging customizable audit report templates can help streamline the auditing process.
7. Keeping comprehensive records of processes
Last but not least, adequate documentation of the entire CAPA procedure is critical, specifically for organizations operating with regulatory requirements. Certainly, having clear records of responsibilities assigned, suggested steps, decisions, and approvals in the CAPA process enhances accountability and traceability.
For instance, during the CAPA process at MediFeck, from issue identification to final closure, several critical documents were documented as mandated by regulatory authorities. The CAPA team formally closed the process and stored all records, such as the investigation report, findings, action plan, implementation records, and training details, on a centralized and secure platform for future reference.
Although proper documentation fosters information sharing and collaboration, it also acts as a reference to study trends and pre-determine quality issues soon. With robust document management and control features, the SharePoint-based solution proves an invaluable tool for streamlining the entire CAPA documentation process.
Expert Insights for Mastering the CAPA Process
As mandated by the regulatory authorities, the CAPA process is the most critical component in your entire quality management efforts. However, many factors, including outdated or inefficient technology, lack of proper procedures, inadequate training, poor root cause analysis, etc., contribute to a failed CAPA process. This leads to recurrent quality issues, compromises customer safety, and increases the risk of non-compliance, ultimately leading to legal repercussions and even damaging brand reputation. Let’s walk through some of the pro tips to successfully implement and maintain an effective CAPA procedure.
Improving communication across cross functional teams: Planning and implementing CAPA is a comprehensive process and involves different stakeholders across the organization. Indeed, there can be a quality assurance team to handle customer complaints, a research and development team that investigates the root cause, and stakeholders from the manufacturing department responsible for implementing changes. However, leveraging a collaborative platform to bring these cross-functional teams together can reduce miscommunication, reduce the risk of recurrence, and contribute to more proactive and innovative problem-solving.
Implement a solution that can be fully customized: While the core principle of the CAPA process remains the same across different industries, you can find significant differences in the terminologies and workflows due to regulatory requirements and operational practices. While a manufacturing industry focuses on non-conformance reports, a food and beverage company may look for HACCP protocols. However, having a fully configurable solution helps organizations personalize their CAPA process. Certainly, it empowers you to create custom workflows, designate approvers accordingly, design custom form templates, and modify the form fields, improving user adoption and overall efficiency.
Leverage automated approval and e-signatures: Manual document routing often leads to non-compliance, lost documents, and delayed responses, specifically in a CAPA process that involves reviews and approvals from multiple stakeholders. However, automating the CAPA procedure enables organizations to smoothen the document workflow, eliminate manual errors, and ensure consistency across the organization. Features offered by SharePoint-based solutions allow organizations to assign one or more approvers and set email notifications to remind stakeholders of pending approval, accelerating the overall CAPA process. Furthermore, integrating electronic signatures into the approval process can create a more efficient, transparent, and compliant CAPA process, even in remote or hybrid environments.
Transform Your CAPA Strategy with BizPortals QMS
Effective CAPA implementation has always been critical for organizations working in a regulatory environment. More than just being complaint, it is imperative to deliver safe and quality products or services to have more and more satisfied customers across the globe.
The complex paper-based processes and outdated legacy systems have turned the overall CAPA processes into a nightmare for many organizations. However, BizPortals QMS, a SharePoint-based quality management system, is all set to revolutionize how organizations interpret the CAPA procedures.
From raising incidents to monitoring process changes and regular audits, you can securely manage documentation on a centralized and fully digital platform. Apart from the robust document control and collaborative features offered by BizPortals QMS, it is a fully configurable solution that offers the flexibility to customize the form templates, workflows, and terminologies that best fit your operations.
Transform your CAPA strategy today! Schedule a demo of BizPortals QMS and see how our solution can streamline your quality management process and ensure compliance.
Get Free Product Tour

